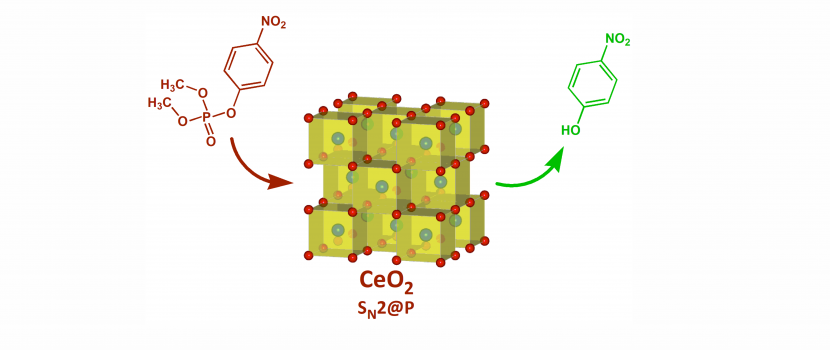
Remarkable properties of cerium dioxide, especially its surface
Cerium dioxide is one of the oxides formed of rare earth elements. It has remarkable physical and chemical properties, particularly the ability to cleave many chemical bonds in hazardous and biologically active substances. This ability is related to the method of its synthesis, as well as the heat treatment of intermediates for its synthesis, especially to the surface properties of its particles. We asked ourselves to what extent and which parameters affect the oxide ability to decompose hazardous substances and if there is any correlation between the selected parameters. The experiments have shown that the CeO2 activity is significantly affected by the relationships between the heat-treatment and the number of surface groups, as well as the particle size of CeO2. The most exciting properties were found for the sample heat-treated at 500 °C, which could decompose 50% of the dangerous substance into harmless compounds within two minutes. While for the other samples, the time was significantly longer. Another benefit of our work is a simple methodology for surface characterization of nanomaterials by classical methods of analytical chemistry, which provide information that is difficult to access by other techniques.
J. Ederer, P. Janoš, M. Šťastný, J. Henych, K. Ederer, M. Šrámová Slušná, J. Tolasz., Nanocrystalline cerium oxide for catalytic degradation of paraoxon methyl: Influence of CeO2 surface properties. Journal of Environmental Chemical Engineering 9 (2021) 106229